Bioavailability Calculator: Measure Absolute and Relative Bioavailability with Clarity
Understanding drug bioavailability is essential for clinical pharmacology, pharmaceutical formulation, and therapeutic dose adjustment. Whether you’re assessing the efficacy of a new oral formulation or comparing two dosage forms, this Bioavailability Calculator simplifies the entire process.
With automatic unit conversions, bar graph visualizations, and instant formula breakdown, this tool is built for both clinical insight and educational depth. It supports both absolute and relative bioavailability modes—essential for drug researchers, formulators, and students.
What Is Bioavailability?
Bioavailability (F) refers to the proportion of an administered drug that reaches systemic circulation in an active form. It is expressed as a percentage and varies significantly between different administration routes and formulations.
There are two types of bioavailability:
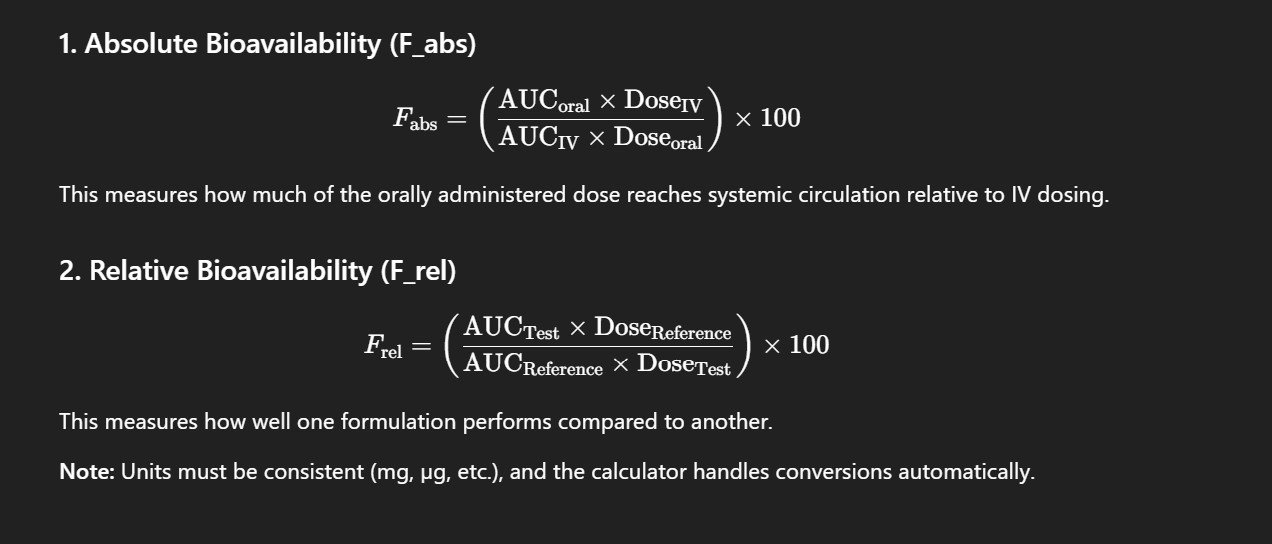
Absolute Bioavailability: Compares the AUC (Area Under Curve) of an extravascular route (like oral) to that of IV administration.
Relative Bioavailability: Compares the AUC of two non-IV formulations (e.g., tablet vs suspension).
Formulas Used in the Calculator
Key Features of the Bioavailability Calculator
Toggle Between Absolute and Relative Modes
At the top, a toggle lets you switch between Absolute and Relative bioavailability. The formula, labels, and fields change accordingly. This flexibility is ideal for comparing both extravascular-to-IV and formulation-to-formulation studies.
Auto Unit Conversion
The tool supports input values in mg or µg. It detects and converts units internally to ensure consistency. You can freely input test and reference doses in different units—no manual conversion needed.
Real-Time Visualization (Chart.js)
A dynamic bar graph visually compares the bioavailability percentage with:
A standard 100% (IV reference)
Reference formulation (in relative mode)
This instantly helps interpret whether the test formulation meets expectations or falls short.
Color-Coded Clinical Interpretation
Once calculated, the result is shown with a color-coded label:
Green (90–110%) → Bioequivalent (ideal range)
Yellow (70–90% or 110–125%) → Acceptable with caution
Red (<70% or >125%) → Outside acceptable limits
These bands are based on regulatory guidelines (e.g., FDA/EMA for generics).
Formula Breakdown with Substituted Values
Every result comes with a full breakdown:
Formula used (absolute or relative)
Substitution of user inputs
Step-by-step multiplication and division
Final result (% bioavailability)
This supports learning and transparency, especially useful for pharmacy students and researchers.
Tooltips for Every Concept
Hovering over the ℹ️ icons gives concise explanations:
What is AUC?
Why IV is the reference?
How does bioavailability affect dosing?
What are regulatory acceptance limits?
This ensures beginners and advanced users alike understand what they’re calculating.
Responsive Layout for All Devices
The layout adjusts for:
Desktop (side-by-side input/result)
Tablets (stacked input and graph)
Mobile (scroll-friendly vertical layout)
Every control, chart, and formula is optimized for small screens, ideal for bedside or fieldwork.
Export Results as PNG or PDF
One click allows exporting the full calculation result (inputs, graphs, formula, interpretation) as:
High-resolution PNG image
Well-formatted PDF document
Perfect for clinical documentation, academic assignments, and regulatory reports.
Instant Reset Button
Need to recalculate? The Reset button clears all fields and output instantly without refreshing the page.
Sample Use Case 1: Absolute Bioavailability
A new oral drug formulation is tested against IV administration. The study provides the following data:
AUC (oral): 120 µg·h/mL
AUC (IV): 150 µg·h/mL
Oral dose: 200 mg
IV dose: 100 mg
Step-by-Step Calculation:
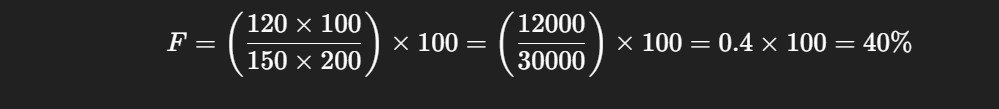
Interpretation:
Only 40% of the orally administered dose reaches systemic circulation, indicating low absolute bioavailability.
Sample Use Case 2: Relative Bioavailability
Two oral formulations are compared: Test (new tablet) vs Reference (marketed capsule).
AUC (Test): 90 µg·h/mL
AUC (Reference): 100 µg·h/mL
Test dose: 150 mg
Reference dose: 150 mg
Step-by-Step Calculation:
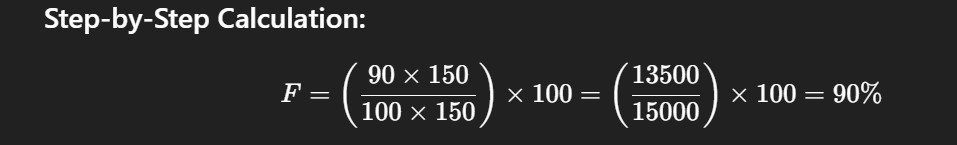
Interpretation:
The test formulation achieves 90% relative bioavailability, which falls within the acceptable range for regulatory approval.
Clinical Importance of Bioavailability
1. Dosing Adjustment
Poor bioavailability may require higher or more frequent dosing. IV drugs have 100% bioavailability, while oral bioavailability varies widely.
2. Generic Drug Approval
Regulatory agencies require relative bioavailability within 80–125% of the reference product for bioequivalence approval.
3. Drug Development
Pharmaceutical companies use bioavailability studies to optimize formulations, improve absorption, and reduce variability.
4. Food-Drug Interactions
Some drugs have increased or decreased absorption when taken with food. Bioavailability studies help assess such effects.
5. Therapeutic Drug Monitoring (TDM)
For narrow therapeutic index drugs (e.g., digoxin, warfarin), understanding and tracking bioavailability ensures safety and efficacy.
Who Should Use This Calculator?
Pharmacy students learning pharmacokinetics
Formulation scientists evaluating dosage designs
Clinicians adjusting drug regimens
Regulatory professionals preparing bioequivalence submissions
Researchers comparing branded vs generic drugs
How to Use the Calculator: Step-by-Step
Toggle Absolute or Relative Mode
Choose based on whether your comparison includes an IV route.Enter AUC and Dose Values
Use mg or µg freely—the calculator handles conversions.Click “Calculate Bioavailability”
Instantly see the calculated value, graph, and interpretation.Review Formula and Explanation
See how your values were substituted and calculated.Export or Reset
Use the PNG/PDF buttons for documentation, or reset to start over.
FAQs
Q1. What is considered good bioavailability?
For oral drugs, 80–125% relative bioavailability is considered acceptable for generics.
Q2. Can I use µg and mg in the same calculation?
Yes. The calculator handles unit consistency automatically.
Q3. What does a bioavailability of 50% mean?
Only half the administered dose reaches systemic circulation. Dose adjustments or reformulation may be needed.
Q4. Why is IV the reference in absolute bioavailability?
IV administration delivers the full dose directly into circulation (100% bioavailability), serving as a gold standard.
Q5. What affects bioavailability?
Factors include:
First-pass metabolism
Drug solubility
Gastric pH
Enzyme induction/inhibition
Formulation design
Q6. Does the tool support IV-to-oral conversion?
Yes, that’s the basis of absolute bioavailability.
Q7. Is this calculator accurate for pediatric dosing?
It calculates correctly, but interpretation in pediatric cases must consider age-based pharmacokinetics.
Q8. Can I use this for injection vs oral suspension comparison?
Yes. Use the relative bioavailability mode for this.
Q9. How can I cite the tool in research?
Export the PNG/PDF result and include a statement such as: “Bioavailability was calculated using the PharmacyFreak Bioavailability Calculator.”
Q10. Is this tool compliant with FDA/EMA standards?
It follows standard formulas used in regulatory guidance but is for educational and clinical support, not a substitute for professional validation.
Conclusion
The Bioavailability Calculator is a comprehensive, responsive, and educational tool that brings clarity to pharmacokinetic calculations. Whether you’re comparing formulations, validating generics, or teaching pharmacology, this tool provides a scientifically sound, visually clear, and user-friendly experience.
Use it confidently to make accurate, interpretable, and well-documented assessments of drug bioavailability.