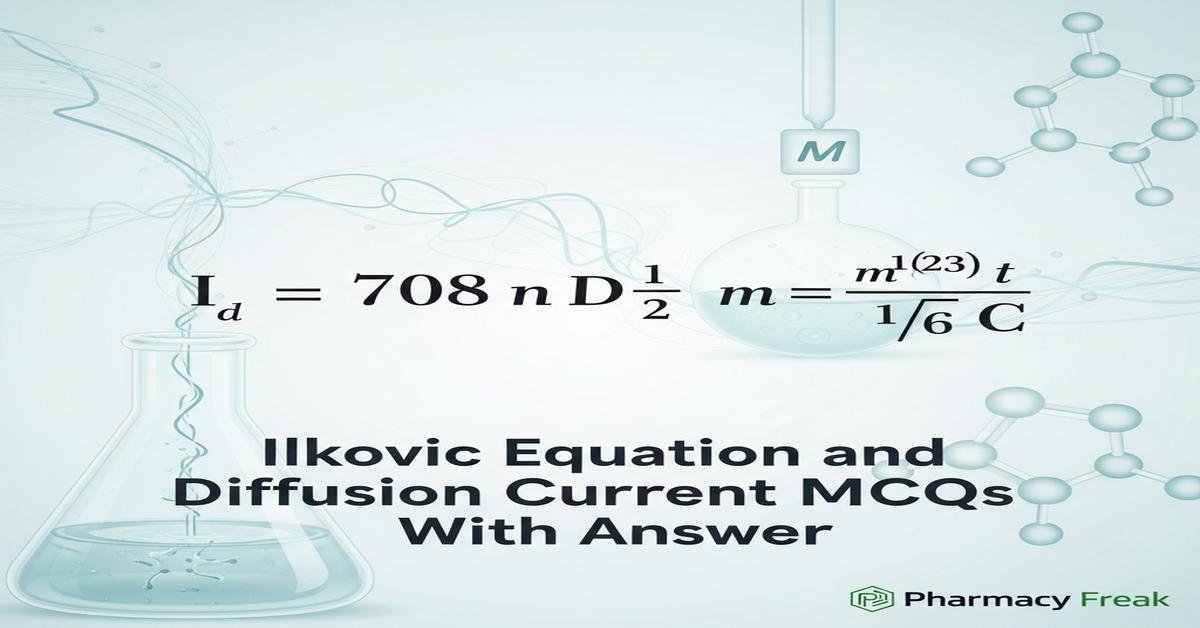
Understanding the Ilkovic equation and diffusion current is essential for B.Pharm students studying analytical electrochemistry and polarography. The Ilkovic equation links diffusion current at a dropping mercury electrode to analyte concentration, diffusion coefficient, mercury flow rate and drop time, enabling quantitative analysis of reducible or oxidizable drugs. The typical Ilkovic expression shows Id proportional to n D^(1/3) m^(2/3) t^(1/6) C, emphasizing diffusion coefficient and electrode hydrodynamics. Key concepts include limiting diffusion current, Ilkovic constant, mass transport (diffusion, convection, migration), and experimental factors such as supporting electrolyte, viscosity and temperature that affect accuracy. Now let’s test your knowledge with 30 MCQs on this topic.
Q1. What does the Ilkovic equation primarily relate in polarography?
- The diffusion current to analyte concentration and physical parameters
- The peak current in cyclic voltammetry to scan rate
- The electrode potential to ionic strength
- The rate of electrode dissolution to stirring speed
Correct Answer: The diffusion current to analyte concentration and physical parameters
Q2. Which electrode is classically used when applying the Ilkovic equation?
- Glassy carbon electrode
- Dropping mercury electrode
- Platinum disk electrode
- Gold microelectrode
Correct Answer: Dropping mercury electrode
Q3. In the standard Ilkovic expression Id ∝ n D^(1/3) m^(2/3) t^(1/6) C, what does “D” represent?
- Diffusion coefficient of the electroactive species
- Dielectric constant of the solvent
- Distance between electrodes
- Density of the mercury drop
Correct Answer: Diffusion coefficient of the electroactive species
Q4. Which exponent is associated with the mercury flow rate (m) in the Ilkovic equation?
- 1/3
- 2/3
- 1/6
- 1
Correct Answer: 2/3
Q5. Which experimental factor is most important to suppress migration and ensure diffusion control in polarographic measurements?
- High ionic strength supporting electrolyte
- Adjusting electrode potential rapidly
- Using a very small electrode area
- Heating the solution significantly
Correct Answer: High ionic strength supporting electrolyte
Q6. What is meant by “diffusion current” in the context of polarography?
- Current limited by the rate at which species diffuse to the electrode surface
- Current caused solely by convection in the cell
- Current due to electrode corrosion
- Current resulting from solution migration under an electric field
Correct Answer: Current limited by the rate at which species diffuse to the electrode surface
Q7. Which variable appears with the smallest exponent in the Ilkovic equation?
- Number of electrons transferred (n)
- Diffusion coefficient (D)
- Drop time (t)
- Mercury flow rate (m)
Correct Answer: Drop time (t)
Q8. How does an increase in solution viscosity generally affect the diffusion current predicted by the Ilkovic equation?
- Increases diffusion current by enhancing mass transport
- Decreases diffusion current by lowering the diffusion coefficient
- No effect because viscosity is not in the Ilkovic equation
- Causes a linear increase in current with viscosity
Correct Answer: Decreases diffusion current by lowering the diffusion coefficient
Q9. Which of the following processes is NOT a mass transport mechanism relevant to polarography?
- Diffusion
- Convection
- Migration
- Electrostriction
Correct Answer: Electrostriction
Q10. The Ilkovic constant is used in the full Ilkovic equation. What does this constant depend on?
- Universal physical constants only
- Units chosen and experimental geometry/conditions
- Only the analyte concentration
- Only the temperature of the lab
Correct Answer: Units chosen and experimental geometry/conditions
Q11. For a diffusion-controlled polarographic wave, the plot of diffusion current (Id) versus analyte concentration (C) is expected to be:
- Linear through the origin
- Parabolic
- Logarithmic
- Exponential
Correct Answer: Linear through the origin
Q12. Which parameter in the Ilkovic equation represents the effective life or duration of a mercury drop?
- n (number of electrons)
- t (drop time)
- D (diffusion coefficient)
- C (concentration)
Correct Answer: t (drop time)
Q13. If the number of electrons transferred in the electrode reaction doubles, the diffusion current predicted by Ilkovic equation will:
- Remain unchanged
- Increase twofold
- Increase fourfold
- Decrease by half
Correct Answer: Increase twofold
Q14. Why is a supporting electrolyte used in polarography when applying the Ilkovic equation?
- To reduce migration and make diffusion the dominant mass transport
- To increase electrode surface area
- To directly increase the diffusion coefficient of analyte
- To change the Ilkovic exponent values
Correct Answer: To reduce migration and make diffusion the dominant mass transport
Q15. Which practical factor must be kept constant to obtain reproducible Ilkovic diffusion currents for concentration analysis?
- Mercury flow rate and drop time
- Distance between the cell walls
- Color of the solution
- Shape of the glassware
Correct Answer: Mercury flow rate and drop time
Q16. What is the typical unit used for the diffusion coefficient (D) in polarographic calculations relevant to Ilkovic equation?
- mol L^-1
- cm^2 s^-1
- A (ampere)
- mg s^-1
Correct Answer: cm^2 s^-1
Q17. Which statement is true about the Ilkovic equation and kinetic control?
- It accounts for slow electrode kinetics explicitly
- It assumes the process is diffusion-controlled, not kinetically limited
- It requires inclusion of electron transfer rate constants
- It applies only when kinetics are the rate-determining step
Correct Answer: It assumes the process is diffusion-controlled, not kinetically limited
Q18. In practice, the measured polarographic current includes background contributions. Which term is typically subtracted to obtain the diffusion current?
- Capacitive current
- Residual or background current
- Faradaic current from the analyte
- Photocurrent
Correct Answer: Residual or background current
Q19. The Ilkovic equation is most directly applicable to which analytical technique?
- Polarography
- Mass spectrometry
- High-performance liquid chromatography (HPLC)
- Infrared spectroscopy
Correct Answer: Polarography
Q20. What does the symbol “m” denote in the Ilkovic equation?
- Mass of analyte in the cell
- Mercury flow rate (mass of mercury per unit time)
- Molarity of supporting electrolyte
- Magnetic field strength
Correct Answer: Mercury flow rate (mass of mercury per unit time)
Q21. How does increasing temperature generally affect the diffusion current, all else equal?
- Decreases current because reactions slow down
- Increases current by increasing the diffusion coefficient
- Has no effect on diffusion current
- Reverses the sign of the current
Correct Answer: Increases current by increasing the diffusion coefficient
Q22. The Stokes–Einstein relation links diffusion coefficient to which other properties?
- Potential and current
- Temperature, viscosity and particle size
- Concentration and pH
- Electrode area and applied potential
Correct Answer: Temperature, viscosity and particle size
Q23. If no supporting electrolyte is present, what deviation from Ilkovic behavior is most likely?
- Enhanced diffusion control with perfect linearity
- Significant migration effects causing nonlinearity
- No current will be observed
- Ilkovic exponents will become integers
Correct Answer: Significant migration effects causing nonlinearity
Q24. Which of the following best describes the “limiting diffusion current” in polarography?
- The maximum current when mass transport by diffusion to the electrode limits the rate
- The instantaneous current during nucleation of mercury drops
- The minimal background current with no analyte
- The current measured at very high overpotential due to gas evolution
Correct Answer: The maximum current when mass transport by diffusion to the electrode limits the rate
Q25. When using Ilkovic equation for quantitative analysis of a drug, the most reliable approach is:
- Directly calculating concentration from a single Id reading without calibration
- Constructing a calibration curve of Id versus known concentrations
- Measuring the peak potential instead of current
- Relying on color change of the solution
Correct Answer: Constructing a calibration curve of Id versus known concentrations
Q26. Which change would most strongly decrease the diffusion current according to the Ilkovic relationship?
- Doubling the analyte concentration
- Reducing diffusion coefficient by a factor of 8
- Doubling the number of electrons transferred
- Doubling the mercury flow rate
Correct Answer: Reducing diffusion coefficient by a factor of 8
Q27. The Ilkovic equation assumes a particular hydrodynamic regime around the drop. Which statement reflects that assumption?
- The diffusion layer is stationary and independent of drop formation
- The drop formation creates a reproducible convective-diffusive flow that the equation accounts for
- There is turbulent mixing at the drop surface
- Mass transport is governed only by bulk stirring
Correct Answer: The drop formation creates a reproducible convective-diffusive flow that the equation accounts for
Q28. Which experimental artifact can most easily distort Ilkovic-derived concentrations if not controlled?
- Ambient light intensity
- Variable mercury drop size or flow rate
- Type of glass used in the beaker
- Color of the electrodes
Correct Answer: Variable mercury drop size or flow rate
Q29. How does the Ilkovic equation treat the number of electrons n in the redox reaction?
- As an inverse factor (Id ∝ 1/n)
- As directly proportional to Id (Id ∝ n)
- It is absent from the equation
- It affects only the potential, not the current
Correct Answer: As directly proportional to Id (Id ∝ n)
Q30. Which of the following is a valid strategy to improve accuracy when using the Ilkovic equation in the lab?
- Ignore background currents since they are negligible
- Maintain constant drop time, mercury flow rate, temperature, and supporting electrolyte
- Reduce supporting electrolyte to allow migration effects
- Vary drop size during calibration to average results
Correct Answer: Maintain constant drop time, mercury flow rate, temperature, and supporting electrolyte

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com