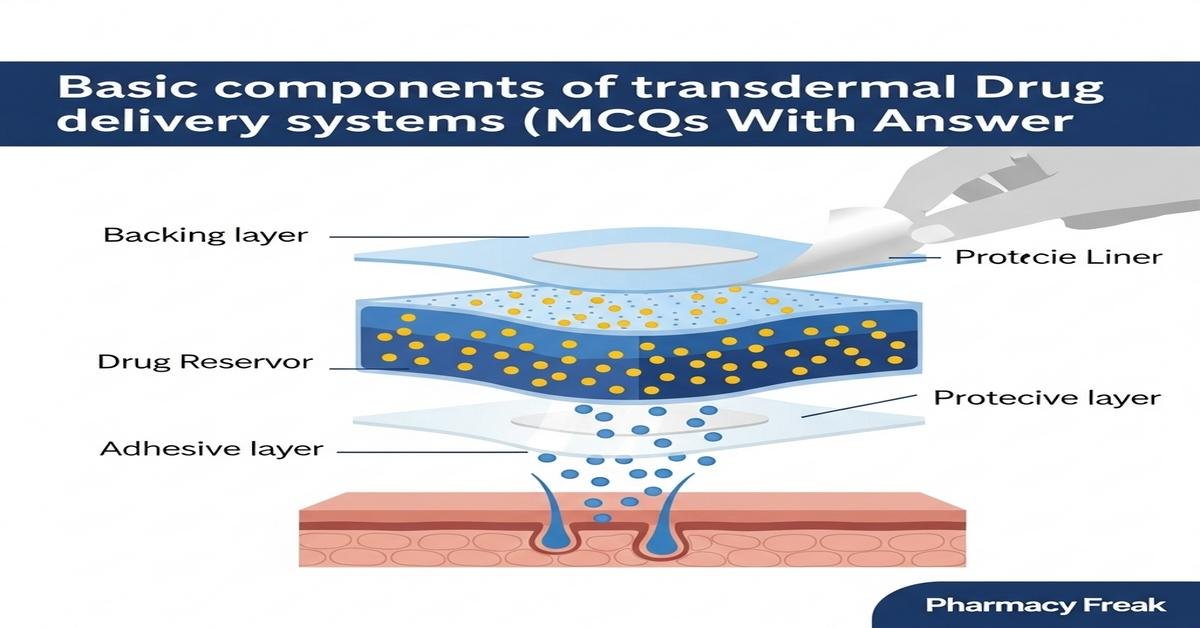
Transdermal drug delivery systems (TDDS) provide controlled systemic delivery of drugs through the skin using specialized patches. Understanding the basic components—backing layer, drug reservoir or matrix, rate-controlling membrane, adhesive layer, release liner, and penetration enhancers—is essential for formulation, evaluation and patient safety. This review emphasizes formulation principles and evaluation methods, including polymer selection, drug loading, skin permeation pathways, adhesive performance, release kinetics and biocompatibility. Knowledge of reservoir versus matrix designs, materials such as acrylics, silicones and polyisobutylene, and tests like in vitro release and peel strength helps B.Pharm students design and assess effective TDDS. Now let’s test your knowledge with 30 MCQs on this topic.
Q1. Which component of a TDDS primarily prevents drug loss and protects the formulation from the external environment?
- Adhesive layer
- Backing layer
- Release liner
- Rate-controlling membrane
Correct Answer: Backing layer
Q2. In a reservoir TDDS, which part contains the drug in a discrete compartment?
- Backing layer
- Release liner
- Drug reservoir
- Adhesive matrix
Correct Answer: Drug reservoir
Q3. Which TDDS design incorporates the drug directly into the pressure-sensitive adhesive?
- Reservoir system
- Matrix (drug-in-adhesive) system
- Transdermal gel system
- Microneedle array
Correct Answer: Matrix (drug-in-adhesive) system
Q4. What is the primary function of a rate-controlling membrane in TDDS?
- Enhance skin penetration
- Control drug release rate from reservoir
- Provide adhesion to skin
- Protect patch during storage
Correct Answer: Control drug release rate from reservoir
Q5. Which class of polymers is commonly used for the backing layer due to flexibility and moisture barrier properties?
- Cellulose ethers
- Polyethylene or polyester films
- Polypeptides
- Hydrogels
Correct Answer: Polyethylene or polyester films
Q6. Which of the following is an example of a chemical penetration enhancer used in TDDS?
- Polyethylene backing
- Oleic acid
- Silicone adhesive
- Polyisobutylene
Correct Answer: Oleic acid
Q7. Which skin layer is the primary barrier to transdermal drug absorption?
- Dermis
- Hypodermis
- Stratum corneum
- Basal layer
Correct Answer: Stratum corneum
Q8. Which pathway is NOT a principal route for drug permeation across the skin?
- Intercellular lipid route
- Transcellular route
- Appendageal (follicular) route
- Vascular lumen route
Correct Answer: Vascular lumen route
Q9. Which property of a drug most favors passive transdermal delivery?
- High molecular weight (>1000 Da)
- Very high hydrophilicity
- Moderate lipophilicity and low molecular weight
- Strong ionization at skin pH
Correct Answer: Moderate lipophilicity and low molecular weight
Q10. Which adhesive type is widely used for drug-in-adhesive TDDS because of skin compatibility?
- Rubber-based adhesive
- Acrylic pressure-sensitive adhesive
- Epoxy resin
- Synthetic polyester adhesive
Correct Answer: Acrylic pressure-sensitive adhesive
Q11. In vitro release testing of TDDS primarily assesses which attribute?
- Skin irritation potential
- Drug release kinetics from the patch
- Patch appearance
- Peel strength
Correct Answer: Drug release kinetics from the patch
Q12. Which test measures the adhesive strength of a patch to human skin or model substrates?
- In vitro permeation test
- Peel strength test
- Differential scanning calorimetry
- pH stability test
Correct Answer: Peel strength test
Q13. Which of the following is a risk associated with reservoir TDDS if the membrane fails?
- Reduced drug release
- Dose-dumping leading to overdose
- Increased patch adhesion
- Improved skin tolerability
Correct Answer: Dose-dumping leading to overdose
Q14. Polyisobutylene is commonly used in TDDS as which component?
- Backing film
- Release liner
- Adhesive elastomer
- Penetration enhancer
Correct Answer: Adhesive elastomer
Q15. Which parameter is critical when selecting a drug candidate for TDDS?
- High daily dose requirement (>100 mg/day)
- Short biological half-life (<1 hour)
- Potent drug with low daily dose and suitable permeability
- Extensive first-pass metabolism only
Correct Answer: Potent drug with low daily dose and suitable permeability
Q16. Which manufacturing step is essential to create a multilayer transdermal patch?
- Lyophilization
- Coating and lamination
- Emulsification only
- Sublimation
Correct Answer: Coating and lamination
Q17. A drug’s partition coefficient (log P) influences which TDDS property most directly?
- Mechanical strength of backing
- Skin permeation and partitioning into stratum corneum
- Patch color
- Adhesive drying time
Correct Answer: Skin permeation and partitioning into stratum corneum
Q18. Which in vitro model is commonly used to evaluate transdermal permeation?
- Franz diffusion cell
- USP disintegration apparatus
- Rotary evaporator
- High-performance liquid chromatography
Correct Answer: Franz diffusion cell
Q19. Why is occlusion by the backing layer important for some TDDS?
- It reduces drug stability
- It increases skin hydration and thereby permeability
- It decreases adhesive strength
- It prevents drug release completely
Correct Answer: It increases skin hydration and thereby permeability
Q20. Which sterilization method is generally avoided for TDDS containing heat-sensitive drugs?
- Gamma radiation
- Ethylene oxide
- Autoclaving (steam sterilization)
- Dry heat at low temperature
Correct Answer: Autoclaving (steam sterilization)
Q21. What is the main role of the release liner in TDDS?
- To control drug release
- To protect the adhesive and drug during storage
- To be applied to the skin directly
- To act as a penetration enhancer
Correct Answer: To protect the adhesive and drug during storage
Q22. Which analytical method is commonly used to quantify drug content in a TDDS patch?
- Nuclear magnetic resonance imaging
- High-performance liquid chromatography (HPLC)
- Thermogravimetric analysis
- Polarimetry
Correct Answer: High-performance liquid chromatography (HPLC)
Q23. Which skin reaction must be evaluated during TDDS development for safety?
- Enhancement index
- Photostability
- Skin irritation and sensitization
- Patch tensile strength
Correct Answer: Skin irritation and sensitization
Q24. Which factor does NOT significantly influence transdermal flux according to Fick’s law?
- Drug concentration gradient
- Diffusion coefficient in skin
- Membrane thickness
- Patch color
Correct Answer: Patch color
Q25. Which of the following is a benefit of matrix TDDS compared to reservoir TDDS?
- Higher risk of dose-dumping
- Simpler design and lower risk of leakage
- Requires rate-controlling membrane
- Impossible to incorporate hydrophilic drugs
Correct Answer: Simpler design and lower risk of leakage
Q26. Silicone adhesives in TDDS are chosen mainly for their:
- High cytotoxicity
- Good skin compatibility and low irritation
- Ability to act as rate-controlling membranes
- Strong hydrophilicity
Correct Answer: Good skin compatibility and low irritation
Q27. Which regulatory requirement is important for TDDS labeling regarding residual drug after use?
- No information is needed
- Instructions for disposal and warnings about residual drug in used patches
- Only manufacturer address is required
- Patch color code
Correct Answer: Instructions for disposal and warnings about residual drug in used patches
Q28. Enhancing skin permeability by increasing skin temperature mainly affects which parameter?
- Drug chemical stability only
- Diffusion coefficient and skin blood flow
- Patch adhesive chemistry
- Backing layer thickness
Correct Answer: Diffusion coefficient and skin blood flow
Q29. Which evaluation assesses whether the drug remains uniformly distributed in the patch matrix?
- Content uniformity testing
- Peel strength test
- Viscosity of adhesive
- Patch folding endurance
Correct Answer: Content uniformity testing
Q30. A TDDS intended for once-weekly dosing most likely requires which characteristic?
- Very rapid initial release and no sustained release
- Prolonged, controlled release and stable drug reservoir
- Extremely high daily drug dose (>500 mg/day)
- No adhesive layer
Correct Answer: Prolonged, controlled release and stable drug reservoir

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com