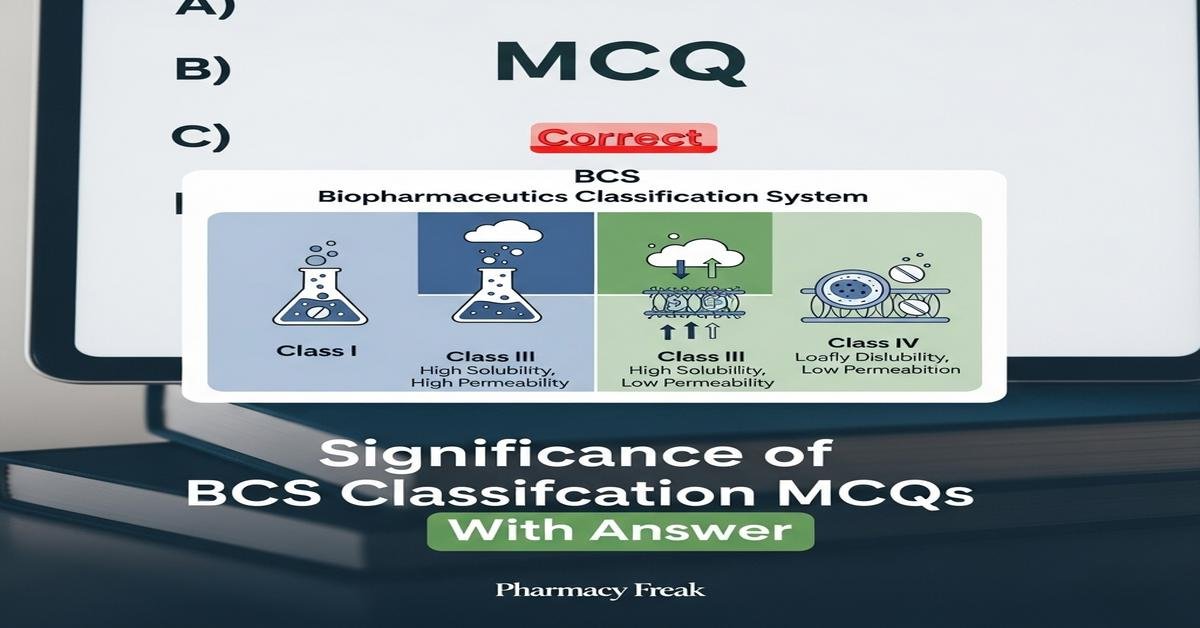
Introduction: The Biopharmaceutics Classification System (BCS) is a cornerstone concept for B. Pharm students, linking drug solubility, permeability, dissolution, and drug absorption to predict oral bioavailability and guide formulation strategies. Understanding BCS classification, dose number, solubility testing, permeability assays (e.g., Caco-2, PAMPA), and regulatory biowaiver criteria equips students to evaluate drug development, generic approval paths, and IVIVC considerations. Mastery of BCS helps in selecting salt forms, solid dispersions, permeability enhancers, and designing dissolution tests that impact bioavailability and therapeutic outcomes. Now let’s test your knowledge with 30 MCQs on this topic.
Q1. What is the primary purpose of the Biopharmaceutics Classification System (BCS)?
- To classify drugs by therapeutic class and dosing frequency
- To categorize drugs by solubility and permeability to predict absorption
- To rank drugs by market sales and patent status
- To identify drug stability under various storage conditions
Correct Answer: To categorize drugs by solubility and permeability to predict absorption
Q2. Which of the following correctly describes a BCS Class II drug?
- High solubility, high permeability
- Low solubility, high permeability
- High solubility, low permeability
- Low solubility, low permeability
Correct Answer: Low solubility, high permeability
Q3. According to common regulatory definitions, a drug is considered “highly soluble” when:
- It is soluble in water at any temperature
- The highest marketed single dose is soluble in 250 mL across pH 1–7.5 at 37°C
- It dissolves completely in 1 L of water
- It has a log P value greater than 3
Correct Answer: The highest marketed single dose is soluble in 250 mL across pH 1–7.5 at 37°C
Q4. “High permeability” in BCS is commonly defined based on:
- In vitro solubility in simulated gastric fluid
- Extent of absorption in humans (commonly ≥85% absorbed)
- Permeability measured only by PAMPA regardless of in vivo data
- Molecular weight below 300 Da
Correct Answer: Extent of absorption in humans (commonly ≥85% absorbed)
Q5. The dose number (Do) is used in BCS to relate dose to solubility. Which formula represents Do?
- Do = (Solubility × Volume) / Dose
- Do = Dose / (Solubility × 250 mL)
- Do = Dose × Solubility
- Do = log(Dose) / log(Solubility)
Correct Answer: Do = Dose / (Solubility × 250 mL)
Q6. Which experimental method is commonly used to measure intrinsic aqueous solubility of a drug substance?
- Caco-2 permeability assay
- Shake-flask solubility method with HPLC analysis
- Mass spectrometry of plasma samples
- PAMPA permeability test
Correct Answer: Shake-flask solubility method with HPLC analysis
Q7. Which in vitro model is a cell-based system widely used to estimate intestinal permeability?
- Shake-flask method
- Caco-2 cell monolayer assay
- PAMPA without cells
- Dissolution apparatus II
Correct Answer: Caco-2 cell monolayer assay
Q8. For BCS-based biowaivers, regulatory agencies most commonly allow waivers for which class of drugs?
- Class IV only
- Class I and certain Class III under strict conditions
- Class II only
- All classes without restrictions
Correct Answer: Class I and certain Class III under strict conditions
Q9. Which BCS class is typically described as “permeability-limited absorption” where solubility is good?
- Class I
- Class II
- Class III
- Class IV
Correct Answer: Class III
Q10. What is a common formulation strategy for a BCS Class II drug to improve oral absorption?
- Use of permeability enhancers only
- Solubility enhancement techniques like micronization, solid dispersions, or cyclodextrins
- Reducing dose without changing formulation
- Adding sustained-release excipients to slow dissolution
Correct Answer: Solubility enhancement techniques like micronization, solid dispersions, or cyclodextrins
Q11. Which factor can make permeability assessment by Caco-2 cells underestimate in vivo absorption?
- Absence of transporters and efflux pumps in Caco-2 cells
- Overexpression of efflux transporters (e.g., P-gp) in the cell model
- Use of 250 mL as dissolution volume
- High drug solubility in buffer
Correct Answer: Overexpression of efflux transporters (e.g., P-gp) in the cell model
Q12. How does drug pKa influence BCS-related solubility?
- pKa determines permeability but not solubility
- Ionic state (ionized vs unionized) across gastrointestinal pH alters apparent solubility
- pKa only affects taste, not dissolution
- pKa always increases solubility irrespective of pH
Correct Answer: Ionic state (ionized vs unionized) across gastrointestinal pH alters apparent solubility
Q13. Which statement correctly contrasts BCS Class I and Class IV drugs?
- Class I: low solubility, high permeability; Class IV: high solubility, low permeability
- Class I: high solubility & permeability; Class IV: low solubility & permeability
- Class I: demands advanced formulation; Class IV: no special formulation needed
- Class I and IV have identical absorption behavior
Correct Answer: Class I: high solubility & permeability; Class IV: low solubility & permeability
Q14. Which in vitro dissolution specification is commonly considered sufficient to support a biowaiver for immediate-release Class I drugs?
- Very slow dissolution in only one medium
- Rapid dissolution (commonly ≥85% in 30 minutes) in relevant media
- No dissolution testing required at all
- Dissolution less than 50% in 60 minutes
Correct Answer: Rapid dissolution (commonly ≥85% in 30 minutes) in relevant media
Q15. What does IVIVC stand for and why is it relevant to BCS?
- In Vitro–In Vivo Correlation; it links dissolution behavior to oral absorption and supports formulation decisions
- In Vivo–In Vitro Calculation; it measures plasma protein binding
- Internal Validation of In Vitro Cells; used only in toxicity testing
- Instant Variation in In Vivo Clearance; used for hepatic metabolism studies
Correct Answer: In Vitro–In Vivo Correlation; it links dissolution behavior to oral absorption and supports formulation decisions
Q16. The Biopharmaceutics Drug Disposition Classification System (BDDCS) differs from BCS mainly by classifying drugs based on:
- Only solubility and pH-solubility profile
- Extent of metabolism and solubility rather than permeability alone
- Color and taste of the drug product
- Manufacturing cost and patent expiry
Correct Answer: Extent of metabolism and solubility rather than permeability alone
Q17. Which analytical consideration is essential when performing shake-flask solubility studies for BCS classification?
- Maintain non-sink conditions intentionally
- Ensure equilibrium is reached and use validated analytical quantification (e.g., HPLC)
- Use only distilled water at room temperature without pH control
- Exclude filtration steps to retain undissolved particles
Correct Answer: Ensure equilibrium is reached and use validated analytical quantification (e.g., HPLC)
Q18. Which statement best explains why some Class III drugs may not qualify for a biowaiver despite high solubility?
- Because Class III drugs are always highly permeable
- Because absorption is permeability-limited and sensitive to excipient changes that can affect transporters or tight junctions
- Because solubility is irrelevant for biowaivers
- Because Class III drugs never dissolve in vitro
Correct Answer: Because absorption is permeability-limited and sensitive to excipient changes that can affect transporters or tight junctions
Q19. Which in vitro permeability assay is cell-free and useful as a high-throughput screen for passive permeability?
- Caco-2 assay
- MDCK cell assay
- PAMPA (Parallel Artificial Membrane Permeability Assay)
- Human intestinal perfusion
Correct Answer: PAMPA (Parallel Artificial Membrane Permeability Assay)
Q20. In BCS context, why are excipients sometimes critical when granting biowaivers for generics?
- Excipients never alter drug performance, so they are ignored
- Certain excipients may affect dissolution, permeability, or transporter activity, altering in vivo absorption
- Only colorants are reviewed because they change taste
- Because excipients increase molecular weight of APIs
Correct Answer: Certain excipients may affect dissolution, permeability, or transporter activity, altering in vivo absorption
Q21. Which parameter most directly determines the rate at which a drug appears in systemic circulation (absorption rate)?
- Tmax only
- Intrinsic dissolution rate, permeability, and gastric emptying
- Only the marketed price
- Color and shape of the tablet
Correct Answer: Intrinsic dissolution rate, permeability, and gastric emptying
Q22. Which process can limit oral bioavailability even if a drug is BCS Class I in vitro?
- Poor tablet aesthetics
- Extensive first-pass metabolism or efflux transport
- High aqueous solubility in all media
- Low molecular weight
Correct Answer: Extensive first-pass metabolism or efflux transport
Q23. For a weakly basic drug with pKa around 6.5, where in the GI tract would you expect higher solubility?
- Lower intestine (higher pH) only
- Stomach (low pH) where the drug is more ionized if basic
- Solubility is identical throughout the GI tract
- Colon only because of microbiota
Correct Answer: Stomach (low pH) where the drug is more ionized if basic
Q24. Which of the following is a correct implication of BCS Class IV drugs?
- They generally pose the least development challenge
- They present both solubility and permeability challenges, often requiring complex formulation strategies
- They are ideal candidates for simple immediate-release tablets without modification
- They always qualify for biowaivers
Correct Answer: They present both solubility and permeability challenges, often requiring complex formulation strategies
Q25. Which regulatory volume is typically used in the dose number calculation for BCS solubility assessment?
- 1000 mL
- 250 mL
- 50 mL
- 10 L
Correct Answer: 250 mL
Q26. When evaluating permeability using human data, which measurement is commonly used to classify a drug as highly permeable?
- Fraction of dose excreted unchanged in urine or absolute oral absorption ≥85%
- Log P less than 0
- Solubility >100 mg/mL only
- In vitro Caco-2 Papp below 0.1 ×10^-6 cm/s
Correct Answer: Fraction of dose excreted unchanged in urine or absolute oral absorption ≥85%
Q27. Which formulation approach is most appropriate for improving absorption of a BCS Class III drug?
- Focus on increasing solubility since permeability is already high
- Modify formulation to avoid excipient interactions that reduce permeability and ensure rapid dissolution
- Ignore dissolution testing because solubility is irrelevant
- Use enteric coating to prevent dissolution in the stomach only
Correct Answer: Modify formulation to avoid excipient interactions that reduce permeability and ensure rapid dissolution
Q28. Which statement about using PAMPA vs Caco-2 assays is accurate?
- PAMPA models active transport and efflux pumps accurately
- Caco-2 is cell-based and can indicate transporter-mediated effects; PAMPA models passive permeability only
- Both assays are identical in mechanism and output
- PAMPA requires living cells and complex culture conditions
Correct Answer: Caco-2 is cell-based and can indicate transporter-mediated effects; PAMPA models passive permeability only
Q29. How can one experimentally demonstrate a drug’s high permeability for regulatory classification when human data are limited?
- Rely solely on log P values
- Use well-validated surrogate data such as mass-balance, human absolute bioavailability, or robust in vitro–in vivo correlation supported by reference compounds
- Assume high permeability if the drug is crystalline
- Classify by color and taste tests
Correct Answer: Use well-validated surrogate data such as mass-balance, human absolute bioavailability, or robust in vitro–in vivo correlation supported by reference compounds
Q30. Calculate the dose number (Do) and solubility classification: A drug has a single highest dose of 50 mg and solubility 0.1 mg/mL. Using 250 mL volume, is it considered highly soluble?
- Do = 0.02; highly soluble
- Do = 2; not highly soluble
- Do = 0.5; highly soluble
- Do = 5; highly soluble
Correct Answer: Do = 2; not highly soluble

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com