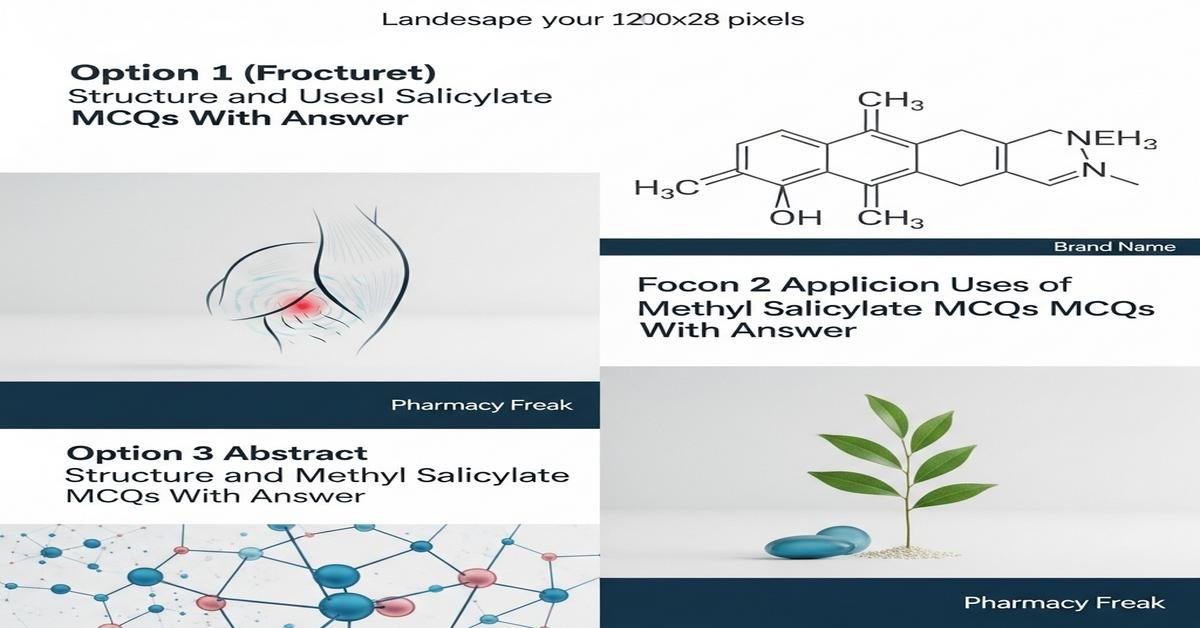
Methyl salicylate is an aromatic ester (methyl 2-hydroxybenzoate) widely studied in pharmaceutical sciences for its structure, pharmacology and formulation uses. B. Pharm students should understand its molecular structure (ester and phenolic OH), synthesis by esterification of salicylic acid, physicochemical properties, analytical identification (IR, NMR, GC), topical formulation roles as a counterirritant/analgesic, metabolic hydrolysis to salicylic acid, and toxicity risks including systemic salicylate poisoning. Familiarity with stability, percutaneous absorption, and regulatory limits is essential for safe formulation and clinical use. This concise review prepares you for practical applications and exam-type questions. Now let’s test your knowledge with 50 MCQs on this topic.
Q1. What is the IUPAC name of methyl salicylate?
- Methyl 2-hydroxybenzoate
- Methyl 3-hydroxybenzoate
- Ethyl 2-hydroxybenzoate
- Methyl salicylaldehyde
Correct Answer: Methyl 2-hydroxybenzoate
Q2. Which functional groups are present in the methyl salicylate molecule?
- Ester and phenolic hydroxyl
- Carboxylic acid and ether
- Ketone and amine
- Alcohol and aldehyde
Correct Answer: Ester and phenolic hydroxyl
Q3. What is the molecular formula of methyl salicylate?
- C7H6O2
- C8H8O3
- C9H10O3
- C8H6O3
Correct Answer: C8H8O3
Q4. Which synthesis method is most commonly used to prepare methyl salicylate from salicylic acid?
- Fischer esterification with methanol and acid catalyst
- Diels–Alder reaction
- Grignard reaction with methyl iodide
- Nitration followed by reduction
Correct Answer: Fischer esterification with methanol and acid catalyst
Q5. Methyl salicylate is primarily obtained from which natural source?
- Wintergreen (Gaultheria procumbens) oil
- Mentha arvensis (mint) leaves
- Citrus peel essential oil
- Clove oil
Correct Answer: Wintergreen (Gaultheria procumbens) oil
Q6. What is the characteristic odor of methyl salicylate?
- Wintergreen-like aroma
- Clove-like aroma
- Minty camphor aroma
- No distinct odor
Correct Answer: Wintergreen-like aroma
Q7. Which statement best describes the solubility of methyl salicylate in water?
- Slightly soluble in water
- Highly soluble in water
- Insoluble in organic solvents
- Completely miscible with water
Correct Answer: Slightly soluble in water
Q8. Which analytical technique is most suitable for quantifying methyl salicylate in a topical formulation?
- Gas chromatography (GC)
- Polarimetry
- Flame photometry
- Ion-selective electrode
Correct Answer: Gas chromatography (GC)
Q9. In IR spectroscopy which prominent peak indicates the ester carbonyl in methyl salicylate?
- Approximately 1700–1735 cm−1
- Approximately 2200 cm−1
- Approximately 1600 cm−1
- Approximately 3400 cm−1
Correct Answer: Approximately 1700–1735 cm−1
Q10. In 1H NMR of methyl salicylate, the methoxy (OCH3) protons typically appear near which chemical shift?
- Around 3.7–4.0 ppm
- Around 0.9–1.2 ppm
- Around 6.5–8.0 ppm
- Around 10–12 ppm
Correct Answer: Around 3.7–4.0 ppm
Q11. Which physiological mechanism explains the topical analgesic effect of methyl salicylate?
- Counterirritant action with local vasodilation and TRP channel activation
- Direct opioid receptor agonism
- Central nervous system opioid release
- Inhibition of acetylcholinesterase at the neuromuscular junction
Correct Answer: Counterirritant action with local vasodilation and TRP channel activation
Q12. What metabolic transformation does methyl salicylate undergo in vivo?
- Hydrolysis by esterases to salicylic acid
- Methylation to form anisole
- Reduction to salicylaldehyde
- Direct conjugation without hydrolysis
Correct Answer: Hydrolysis by esterases to salicylic acid
Q13. Which adverse effect is most associated with excessive systemic absorption of methyl salicylate?
- Salicylate poisoning leading to tinnitus, metabolic acidosis
- Cholinergic crisis with muscle weakness
- Severe hypertension and bradycardia
- Acute renal failure due to crystal deposition
Correct Answer: Salicylate poisoning leading to tinnitus, metabolic acidosis
Q14. Why are methyl salicylate-containing products contraindicated in young children?
- Risk of Reye’s syndrome and salicylate toxicity
- Causes permanent staining of teeth
- Leads to growth retardation
- Enhances risk of diabetes
Correct Answer: Risk of Reye’s syndrome and salicylate toxicity
Q15. Which formulation types commonly contain methyl salicylate as an active ingredient?
- Liniments, balms, creams and ointments for topical analgesia
- Oral tablets for systemic analgesia
- Eye drops for conjunctivitis
- Injectable solutions for intramuscular use
Correct Answer: Liniments, balms, creams and ointments for topical analgesia
Q16. What effect does occlusive dressing or heat application have on topical methyl salicylate absorption?
- Increases percutaneous absorption significantly
- Prevents absorption entirely
- Has no effect on absorption
- Degrades the molecule rendering it inactive
Correct Answer: Increases percutaneous absorption significantly
Q17. Which statement regarding the stability of methyl salicylate is correct?
- It can hydrolyze under acidic or basic conditions to salicylic acid and methanol
- It is completely inert to hydrolysis under all conditions
- It polymerizes rapidly on standing at room temperature
- It oxidizes to form salicylaldehyde under neutral pH
Correct Answer: It can hydrolyze under acidic or basic conditions to salicylic acid and methanol
Q18. Which safety precaution is most important when handling concentrated methyl salicylate in the lab?
- Avoid skin contact and inhalation; use gloves and fume hood
- Store under strong alkaline solution to stabilize
- Keep in direct sunlight to prevent degradation
- No special precautions required
Correct Answer: Avoid skin contact and inhalation; use gloves and fume hood
Q19. Which analytical method best separates methyl salicylate from other volatile components in an essential oil?
- Gas chromatography–mass spectrometry (GC–MS)
- Polarimetry
- Thermogravimetric analysis
- Conductivity measurement
Correct Answer: Gas chromatography–mass spectrometry (GC–MS)
Q20. Which IR band would indicate the presence of the phenolic OH in methyl salicylate?
- A broad band around 3200–3600 cm−1
- Sharp peak at 2250 cm−1
- Strong band at 1700 cm−1 only
- No OH stretching is observed
Correct Answer: A broad band around 3200–3600 cm−1
Q21. Which of the following is the primary reason methyl salicylate can cause systemic anticoagulant effects?
- Metabolic conversion to salicylic acid that can potentiate bleeding
- Direct inhibition of vitamin K epoxide reductase
- Chelation of calcium in blood
- Stimulation of platelet aggregation
Correct Answer: Metabolic conversion to salicylic acid that can potentiate bleeding
Q22. What is a common concentration range for methyl salicylate in over-the-counter topical analgesic products?
- Approximately 10–30% w/w
- 60–80% w/w
- 0.001–0.01% w/w
- 100% pure without dilution
Correct Answer: Approximately 10–30% w/w
Q23. Which statement about percutaneous toxicity of methyl salicylate is true?
- Application over large skin areas or under heat can produce systemic salicylate toxicity
- Topical application never leads to systemic absorption
- It is only toxic if ingested, not when applied topically
- Skin absorption is negligible even with repeated use
Correct Answer: Application over large skin areas or under heat can produce systemic salicylate toxicity
Q24. Which reagent is typically used to catalyze esterification of salicylic acid with methanol to form methyl salicylate?
- Sulfuric acid
- Sodium hydroxide
- Potassium permanganate
- Hydrazine
Correct Answer: Sulfuric acid
Q25. Which biological assay or sign is commonly associated with early salicylate toxicity?
- Tinnitus (ringing in ears)
- Hyperpigmentation of skin
- Severe hypothermia without other symptoms
- Profuse watery diarrhea only
Correct Answer: Tinnitus (ringing in ears)
Q26. Which solvent is most appropriate for extracting methyl salicylate from plant material or essential oil for analysis?
- Diethyl ether or dichloromethane
- Distilled water only
- Strong aqueous acid
- Concentrated sodium hydroxide solution
Correct Answer: Diethyl ether or dichloromethane
Q27. Which chromatographic mode is commonly used for HPLC analysis of methyl salicylate in formulations?
- Reversed-phase HPLC with C18 column
- Chiral HPLC with cellulose column
- Normal-phase HPLC with silica and pure hexane mobile phase
- Size-exclusion chromatography
Correct Answer: Reversed-phase HPLC with C18 column
Q28. From a chemical classification standpoint, methyl salicylate is best described as:
- An aromatic ester (salicylate ester)
- A saturated aliphatic alcohol
- A primary amide
- An aliphatic ketone
Correct Answer: An aromatic ester (salicylate ester)
Q29. Which enzymatic family is primarily responsible for hydrolyzing methyl salicylate to salicylic acid in tissues?
- Carboxylesterases (esterases)
- Cytochrome P450 oxidases
- Kinases
- Lipoxygenases
Correct Answer: Carboxylesterases (esterases)
Q30. Why is methyl salicylate considered a counterirritant rather than a true systemic analgesic when applied topically?
- It produces local irritation and increased blood flow that distracts from deeper pain
- It blocks sodium channels in peripheral nerves systemically
- It acts as a central opioid receptor agonist
- It permanently destroys nociceptors at application site
Correct Answer: It produces local irritation and increased blood flow that distracts from deeper pain
Q31. Which of the following is the principal reason methyl salicylate may be restricted or labeled with warnings in OTC products?
- Risk of systemic salicylate toxicity if misused
- High potential for fostering antibiotic resistance
- Marked teratogenicity with topical use
- Rapid explosive decomposition at room temperature
Correct Answer: Risk of systemic salicylate toxicity if misused
Q32. Which reaction occurs when methyl salicylate is treated with aqueous sodium hydroxide?
- Saponification yielding sodium salicylate and methanol
- Reduction to salicylaldehyde
- Nitration to form nitro-salicylate
- Elimination to produce phenol
Correct Answer: Saponification yielding sodium salicylate and methanol
Q33. Which of the following best describes the physical state of pure methyl salicylate at room temperature?
- Viscous, colorless oily liquid
- White crystalline solid
- Colorless gaseous vapor at STP
- Powdery hygroscopic solid
Correct Answer: Viscous, colorless oily liquid
Q34. Which safety symptom should prompt immediate medical evaluation after significant dermal exposure to methyl salicylate?
- Persistent vomiting, tinnitus, hyperventilation
- Mild transient itching only
- Short-term mild warmth at application site
- Temporary yellowing of superficial skin layers
Correct Answer: Persistent vomiting, tinnitus, hyperventilation
Q35. Which regulatory consideration is important when formulating topical products with methyl salicylate?
- Limiting concentration and including child-safety warnings
- Ensuring it exceeds 50% to be effective
- Omitting any label regarding systemic absorption risks
- Labeling as safe for ingestion in small amounts
Correct Answer: Limiting concentration and including child-safety warnings
Q36. Which of the following is a common co-active ingredient with methyl salicylate in topical analgesic rubs?
- Menthol
- Penicillin
- Atropine
- Insulin
Correct Answer: Menthol
Q37. What is the typical boiling point range of methyl salicylate at atmospheric pressure?
- Approximately 220 °C
- Approximately 78 °C
- Approximately 56 °C
- Approximately 350 °C
Correct Answer: Approximately 220 °C
Q38. Which statement correctly describes the acidity/basicity of methyl salicylate?
- Overall neutral ester with a phenolic OH that is weakly acidic
- Strongly basic due to a tertiary amine group
- Strong mineral acid
- Strongly alkaline and corrosive
Correct Answer: Overall neutral ester with a phenolic OH that is weakly acidic
Q39. Which laboratory method is typically used to confirm identity of methyl salicylate by molecular mass?
- Gas chromatography–mass spectrometry (GC–MS)
- Ultraviolet-visible spectrophotometry only
- Polarimetry to determine optical rotation
- Colorimetric pH paper test
Correct Answer: Gas chromatography–mass spectrometry (GC–MS)
Q40. Which precaution is important when combining methyl salicylate-containing topical products with oral anticoagulants?
- Monitor for increased bleeding risk due to salicylate interactions
- No precautions necessary as topical products never interact
- Recommend doubling oral anticoagulant dose
- Advise concurrent ingestion of vitamin K to prevent interaction
Correct Answer: Monitor for increased bleeding risk due to salicylate interactions
Q41. Which chemical process best describes conversion of methyl salicylate to salicylic acid in the presence of water and acid or base?
- Hydrolysis (ester cleavage)
- Oxidative decarboxylation
- Friedel–Crafts acylation
- Hydrogenation
Correct Answer: Hydrolysis (ester cleavage)
Q42. Which occupational exposure control is most appropriate for workers handling neat methyl salicylate?
- Local exhaust ventilation and dermal protection
- Storage at high temperature to prevent condensation
- Continuous ingestion of small amounts to develop tolerance
- No controls required; it is nonhazardous
Correct Answer: Local exhaust ventilation and dermal protection
Q43. Which symptom is NOT typically associated with chronic salicylate toxicity?
- Chronic hypertrichosis (excessive hair growth)
- Persistent tinnitus
- Chronic headache and dizziness
- Gastrointestinal irritation
Correct Answer: Chronic hypertrichosis (excessive hair growth)
Q44. What is the recommended first-line decontamination for significant oral ingestion of methyl salicylate if presenting early?
- Activated charcoal administration
- Induce vomiting at home
- Give milk to neutralize toxin
- No decontamination; observe only
Correct Answer: Activated charcoal administration
Q45. Which lab finding is characteristic of significant salicylate poisoning?
- Mixed respiratory alkalosis and metabolic acidosis
- Isolated metabolic alkalosis without respiratory changes
- Severe hyperkalemia only
- Profound hypoglycemia without acid–base disturbance
Correct Answer: Mixed respiratory alkalosis and metabolic acidosis
Q46. Which of the following is an appropriate storage condition for methyl salicylate in the pharmacy?
- Cool, well-ventilated area away from heat and flames
- Open container in direct sunlight
- Store near strong oxidizers
- Freeze below −20 °C for stability
Correct Answer: Cool, well-ventilated area away from heat and flames
Q47. Which change in formulation would most reduce percutaneous absorption of methyl salicylate?
- Use of a less occlusive topical base (non-occlusive cream)
- Applying under a heat pack to the area
- Using alcohol-rich solvent to enhance penetration
- Applying thick occlusive dressing over the product
Correct Answer: Use of a less occlusive topical base (non-occlusive cream)
Q48. Which statement about using methyl salicylate during pregnancy is most prudent?
- Avoid extensive or high-concentration topical use without medical advice
- It is safe for unlimited use in pregnancy
- Oral ingestion is preferred over topical application
- It is a recommended supplement during pregnancy
Correct Answer: Avoid extensive or high-concentration topical use without medical advice
Q49. Which chemical test would indicate presence of free salicylic acid resulting from hydrolysis of methyl salicylate?
- Ferric chloride test producing violet coloration
- Benedict’s test producing a red precipitate
- Tollens’ test giving silver mirror
- Biuret test forming purple complex
Correct Answer: Ferric chloride test producing violet coloration
Q50. What is the primary industrial method to obtain methyl salicylate from plant material like wintergreen leaves?
- Steam distillation of plant material to collect essential oil
- Cold maceration in aqueous saline solution only
- Direct solvent-free microwave synthesis in the field
- Supercritical CO2 extraction yielding inorganic salts
Correct Answer: Steam distillation of plant material to collect essential oil

I am a Registered Pharmacist under the Pharmacy Act, 1948, and the founder of PharmacyFreak.com. I hold a Bachelor of Pharmacy degree from Rungta College of Pharmaceutical Science and Research. With a strong academic foundation and practical knowledge, I am committed to providing accurate, easy-to-understand content to support pharmacy students and professionals. My aim is to make complex pharmaceutical concepts accessible and useful for real-world application.
Mail- Sachin@pharmacyfreak.com