Table of Contents
Introduction
Azathioprine is an immunosuppressive and cytotoxic drug primarily used in organ transplantation, autoimmune disorders, and inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus (SLE), and inflammatory bowel disease (IBD).
Mechanism of Action of Azathioprine involves suppression of DNA synthesis and lymphocyte proliferation by interfering with purine metabolism. It acts as a prodrug of 6-mercaptopurine (6-MP), a purine analogue that inhibits nucleic acid synthesis, thereby suppressing the immune response.
Azathioprine helps prevent graft rejection and autoimmune tissue destruction by selectively targeting rapidly dividing immune cells.
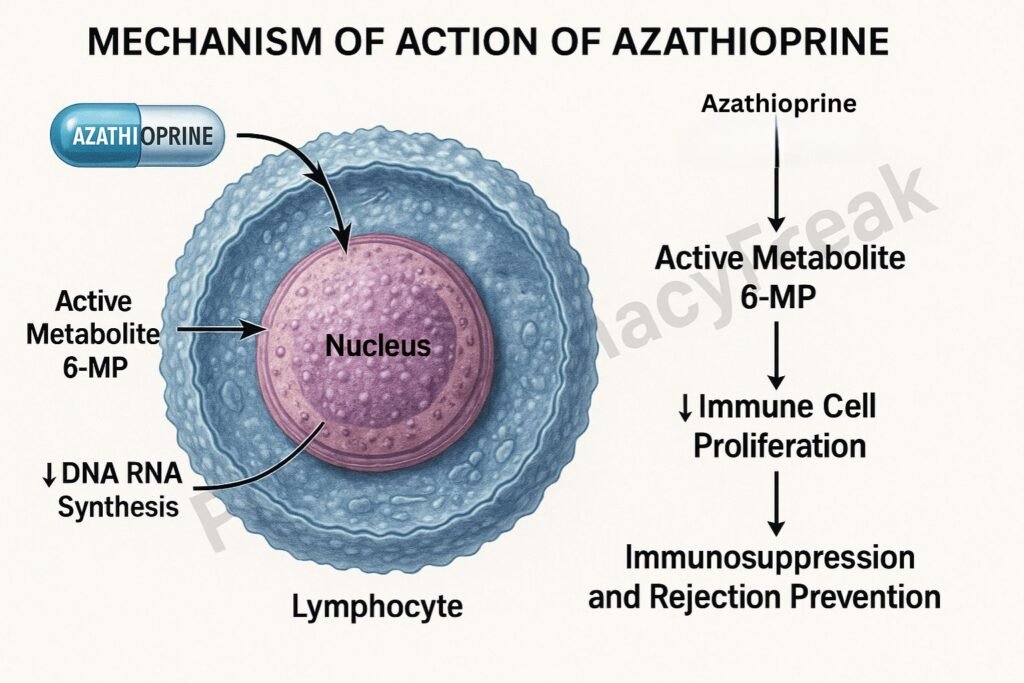
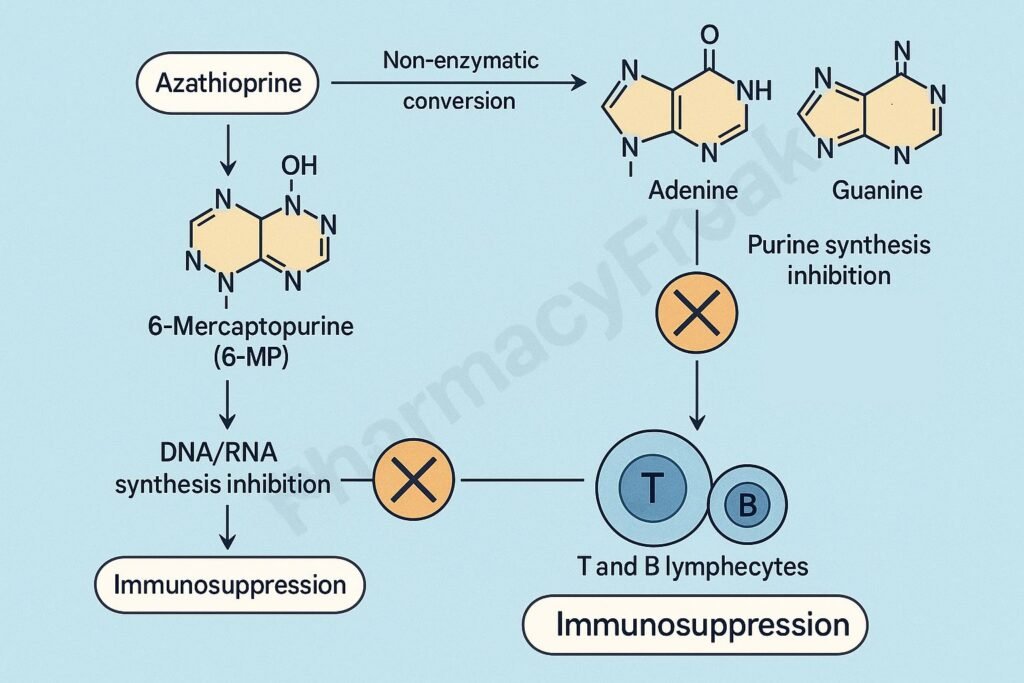
Mechanism of Action (Step-wise)
1. Conversion to Active Metabolite
- Mechanism:
- Azathioprine is a prodrug that is non-enzymatically converted into 6-mercaptopurine (6-MP) in the body.
- 6-MP is then metabolized into active thiopurine nucleotides such as:
- 6-thioinosinic acid (TIMP)
- 6-thioguanine nucleotides (6-TGNs)
- Effect:
- These metabolites are incorporated into DNA and RNA, disrupting nucleic acid synthesis and function.
2. Inhibition of Purine Nucleotide Synthesis
- Mechanism:
- Active metabolites of 6-MP inhibit de novo purine synthesis, a crucial process for DNA and RNA formation in proliferating cells.
- Specifically, azathioprine inhibits enzymes such as:
- Amidophosphoribosyl transferase (rate-limiting enzyme of purine synthesis).
- This leads to depletion of adenine and guanine nucleotides.
- Effect:
- ↓ DNA and RNA synthesis → ↓ proliferation of immune cells (T and B lymphocytes).
3. Suppression of Lymphocyte Proliferation
- Mechanism:
- Lymphocytes (especially activated T cells) rely on de novo purine synthesis for proliferation.
- By depleting purine pools, azathioprine selectively suppresses immune cell replication without significantly affecting resting cells.
- Effect:
- ↓ T-cell activation
- ↓ B-cell antibody production
- ↓ Cytokine release and immune response
4. Cytotoxic Incorporation into Nucleic Acids
- Mechanism:
- 6-thioguanine nucleotides are incorporated into DNA and RNA, causing strand breaks, faulty base pairing, and apoptosis in proliferating immune cells.
- Effect:
- Induces immunosuppression through selective cytotoxicity toward lymphoid tissue.
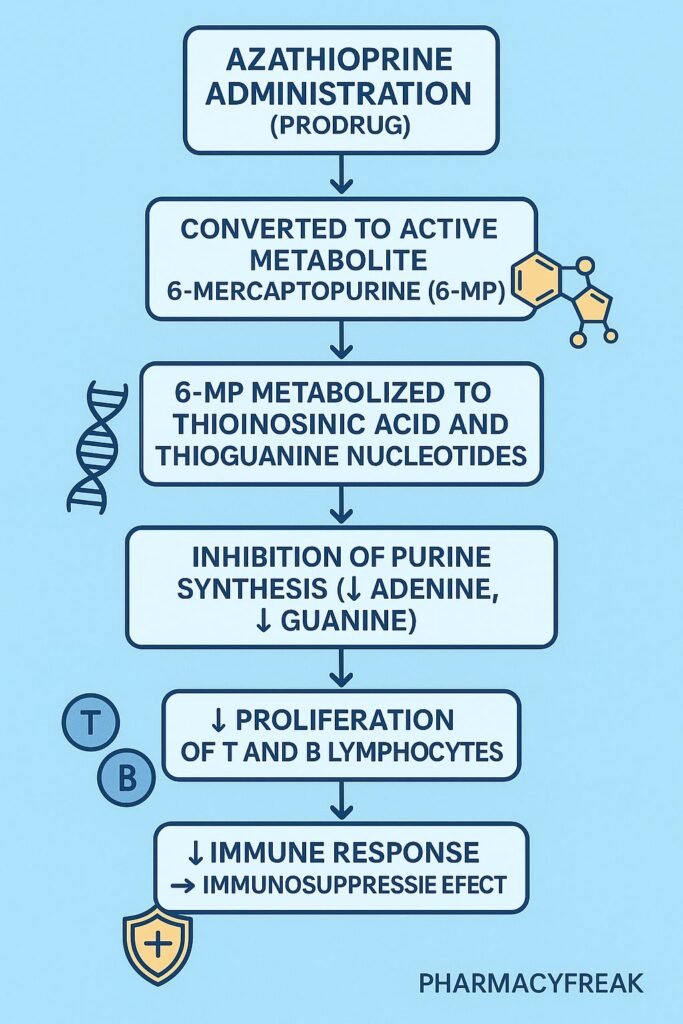
5. Overall Immunosuppressive Effect
| Target | Action of Azathioprine | Outcome |
|---|---|---|
| T lymphocytes | Inhibits activation and proliferation | ↓ Cellular immunity |
| B lymphocytes | Reduces antibody production | ↓ Humoral immunity |
| Cytokine response | Suppressed | ↓ Inflammation |
| Graft rejection | Prevented | Improved transplant survival |
Pharmacokinetics
- Absorption: Well absorbed orally.
- Onset of Action: Slow (2–6 weeks).
- Metabolism:
- Converted to 6-MP by glutathione-S-transferase in liver and RBCs.
- Further metabolized by:
- Thiopurine methyltransferase (TPMT) → inactive metabolites.
- Xanthine oxidase (XO) → inactive metabolites.
- Genetic polymorphism of TPMT affects drug toxicity.
- Excretion: Renal (as metabolites).
Clinical Uses
- Organ transplantation: Prevention of graft rejection (especially kidney and liver).
- Autoimmune diseases:
- Rheumatoid arthritis
- Systemic lupus erythematosus (SLE)
- Autoimmune hepatitis
- Myasthenia gravis
- Inflammatory bowel disease (Crohn’s disease, ulcerative colitis)
- Dermatologic conditions: Pemphigus vulgaris, psoriasis (second-line).
Adverse Effects
- Hematologic: Bone marrow suppression (leukopenia, anemia, thrombocytopenia).
- Gastrointestinal: Nausea, vomiting, pancreatitis, hepatotoxicity.
- Infectious risk: Increased susceptibility to infections.
- Neoplasia: Long-term use increases risk of lymphomas and skin cancers.
- Drug interaction:
- Allopurinol (xanthine oxidase inhibitor) increases azathioprine toxicity — dose should be reduced to 25–33%.
Comparative Table
| Parameter | Azathioprine | Methotrexate | Mycophenolate mofetil |
|---|---|---|---|
| Mechanism | Inhibits purine synthesis via 6-MP | Inhibits dihydrofolate reductase | Inhibits IMP dehydrogenase |
| Target Cells | T & B lymphocytes | Rapidly dividing cells | Lymphocytes (de novo pathway) |
| Onset of Action | 2–6 weeks | 1–2 weeks | 1–2 weeks |
| Main Use | Transplant, autoimmune diseases | Rheumatoid arthritis, psoriasis | Transplant, autoimmune disease |
| Toxicity | Myelosuppression, hepatotoxicity | Hepatotoxicity, mucositis | GI upset, cytopenia |
MCQs
1. Azathioprine is a prodrug of:
a) 6-Thioguanine
b) 6-Mercaptopurine
c) Adenosine
d) Methotrexate
Answer: b) 6-Mercaptopurine
2. The primary mechanism of azathioprine involves inhibition of:
a) Pyrimidine synthesis
b) Purine synthesis
c) Protein synthesis
d) DNA methylation
Answer: b) Purine synthesis
3. Azathioprine suppresses immunity by:
a) Increasing IL-2 synthesis
b) Blocking T and B cell proliferation
c) Stimulating cytokine release
d) Enhancing macrophage activity
Answer: b) Blocking T and B cell proliferation
4. The enzyme responsible for azathioprine activation is:
a) Xanthine oxidase
b) Glutathione-S-transferase
c) Monoamine oxidase
d) Adenosine deaminase
Answer: b) Glutathione-S-transferase
5. Bone marrow suppression due to azathioprine is enhanced by:
a) Aspirin
b) Allopurinol
c) Propranolol
d) Amlodipine
Answer: b) Allopurinol
6. The key site of azathioprine action in the immune system is:
a) Macrophages
b) Lymphocytes
c) Platelets
d) Endothelial cells
Answer: b) Lymphocytes
7. Genetic deficiency of which enzyme increases azathioprine toxicity?
a) TPMT (Thiopurine methyltransferase)
b) G6PD
c) COMT
d) Dihydropyrimidine dehydrogenase
Answer: a) TPMT (Thiopurine methyltransferase)
8. Azathioprine is used to prevent:
a) Thrombosis
b) Graft rejection
c) Bronchospasm
d) Gastric acid secretion
Answer: b) Graft rejection
9. Which of the following is a delayed adverse effect of azathioprine?
a) Nephrotoxicity
b) Lymphoma
c) Hypotension
d) Bradycardia
Answer: b) Lymphoma
10. The immunosuppressive effect of azathioprine is mainly due to:
a) Activation of macrophages
b) Inhibition of purine synthesis and lymphocyte proliferation
c) Blocking histamine receptors
d) Inhibition of cyclooxygenase
Answer: b) Inhibition of purine synthesis and lymphocyte proliferation
FAQs
Q1. How does azathioprine differ from corticosteroids?
Azathioprine directly suppresses lymphocyte proliferation by blocking DNA synthesis, while corticosteroids suppress cytokine production and immune activation.
Q2. Why should azathioprine dose be reduced with allopurinol?
Because allopurinol inhibits xanthine oxidase, which metabolizes 6-MP. Co-administration increases 6-MP toxicity and bone marrow suppression.
Q3. How long does azathioprine take to work in autoimmune diseases?
The immunosuppressive effect develops gradually over 4–6 weeks due to its action on proliferating lymphocytes.
Q4. What monitoring is required during azathioprine therapy?
Regular CBC (complete blood count) and liver function tests to detect myelosuppression and hepatotoxicity.
Q5. Is azathioprine safe in pregnancy?
Generally avoided, but may be used in transplant or severe autoimmune cases under supervision; it’s classified as pregnancy category D.
References

I am pursuing MBA in pharmaceutical management from NIPER Hyderabad with a strong academic record and proven success in national-level pharmacy entrance exams. I secured AIR 61 in NIPER 2024 (MS/M.Pharm) and AIR 27 in NIPER MBA, along with AIR 147 in GPAT 2024 and AIR 907 in GPAT 2023. I also achieved AIR 6 in AIIMS CRE-2025 for Drug Store Keeper and was selected as a Pharmacist (AIR 61) for ESIC. Additionally, I was the Runner-Up in Round 2 of the EY Case Study Competition.
At PharmacyFreak.com, I aim to guide future pharmacists through expert content, exam strategies, and insightful resources based on real experience and academic excellence.
Mail- harsh@pharmacyfreak.com

